Background: Over the past 30 years, the incidence of acute myelogenous leukemia (AML) has progressively increased across the globe, and until recently, therapeutic options were primarily limited to the standard “7 + 3” cytotoxic chemotherapy. The economic burden associated with the management of AML has steadily increased and significantly escalated with the introduction of new targeted therapies. To our knowledge, there are very few studies that have looked at the major drivers of cost in AML treatment over the past decade and how they have evolved.
Methods: We conducted a retrospective review with IRB approval of all the medical costs associated with the initial course of chemotherapy, administered in the hospital setting, in newly diagnosed AML patients at the Georgia Cancer Center (GCC) from 2011 to 2022. All medical costs were obtained through the accounting system at Augusta University Medical Center (AUMC) using the Patient Billing Department. Every direct cost from the patient's first to last day of hospitalization during which they received induction was included.
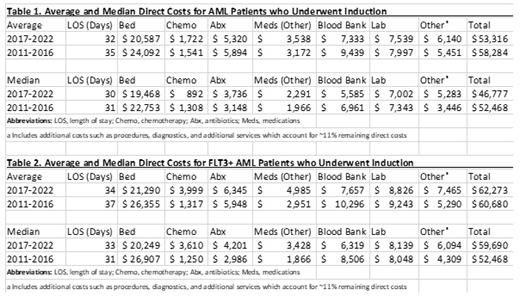
Results: A total of 183 patients were identified. Patients were excluded for the following reasons: 3 patients left to receive induction elsewhere, and 2 patients left against medical advice for unclear reasons. A total of 178 newly diagnosed AML patients fit the above criteria during this period with a median age of 61 years. The median length of stay (LOS) of these 178 patients was 30 days. Between 2011 to 2016, 71 patients were treated and had a median age of 65 years, while 107 patients were treated between 2017 to 2022 with a median age of 57 years. Major drivers of total direct cost were identified which included inpatient bed (~40%), medications (~20%), blood bank products (~15%), and lab tests (~14%). Medication costs were further reviewed with breakdown as follows: chemotherapy (~3%), antibiotics (~10%), and all other medications (~7%). These medication costs were based on 340B pricing. The average and median direct costs from the above categories were compared from 2011-2016 to 2017-2022 (Table 1). The 2017-2022 group had significantly lower median and average bed cost, blood bank product cost, and overall total cost. The LOS was also noted to be shorter in the 2017-2022 group. We then analyzed the subset of patients (n=64) who received standard induction chemotherapy and excluded patients who required reinduction (n=8) or who died during induction (n=13). The same trends were identified in this subset analysis. AML patients with FLT3 mutations, which consisted of 41 of the total 178 patients, were of particular interest given the increased number of targeted therapies that have been approved since 2017. This was investigated further by comparing the direct average and median costs of the categories noted above between the 2011-2016 group with 11 FLT3+ AML patients to the 2017-2022 group with 30 FLT3+ AML patients (Table 2). The average and median medication costs, including chemotherapy, antibiotics, and all other medications, were higher in the 2017-2022 group. This resulted in the total direct cost being higher in the 2017-2022 group despite having lower blood bank and inpatient bed costs.
Conclusion: Over the past decade, many significant changes have occurred in several of the driving factors associated with the cost of AML therapy. Still, there has been a decrease in length of stay, inpatient bed costs, blood bank costs, and overall total costs from 2017-2022 compared to 2011-2016. In the subset of FLT3+ AML patients, there was a shift in the financial landscape with an increase in medication costs that influenced the overall cost. It is likely that the economic impact from this will continue to grow as novel therapies become more available and the number of patients being treated continues to increase.
Disclosures
Keruakous:BMS: Membership on an entity's Board of Directors or advisory committees; Janssen: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding. Cortes:Gilead: Consultancy; Novartis: Consultancy, Research Funding; Forma Therapuetic: Consultancy; Takeda: Consultancy, Honoraria; Abbvie: Consultancy, Research Funding; Biopath Holdings: Consultancy, Current holder of stock options in a privately-held company, Membership on an entity's Board of Directors or advisory committees, Research Funding; Pfizer: Consultancy, Research Funding. Kota:Incyte: Research Funding; Pfizer: Honoraria; Kite: Honoraria; Novartis: Honoraria.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal